

Registered Products :
Inestom 1G/10ML, levocarnitine (L-Carnitine ) vial -
Oral solution single dose

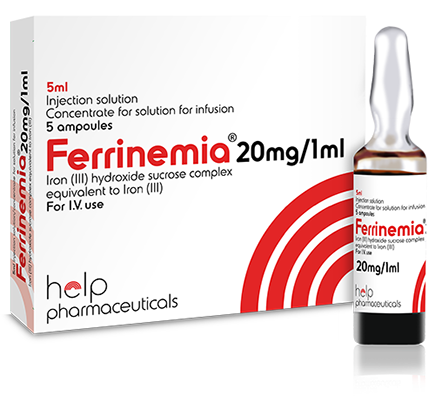
- Iron(III)Injection 20mg/1ml (Ferrinemia),5ml,amp
Products for near future :
Raw materials and Supplements
® Inestom
LEVOCARNITINE
Oral solution single dose 1g/10ml & 2g/10ml
®NAME OF THE MEDICINAL PRODUCT: Inestom
COMPOSITION:
• Active ingredient: Levocarnitine (L-carnitine).
• Excipients of the 1g/10ml Oral Solution: Malic acid, Methylparaben, Propylparaben, Sodium saccharin dihydrate, Orange flavour, Purified water.
• Excipients of the 2g/10ml Oral Solution: Malic acid, Sodium benzoate, Sodium saccharin dihydrate, Pineapple flavour, Purified water.
DRUG FORMULATION:
Oral Solution single dose.
CONCENTRATION OF THE ACTIVE INGREDIENT:
Oral solution single dose: Each vial contains Levocarnitine 1g or 2g.
PRESENTATION:
Oral Solutions single dose: Box containing 10 vials of 10 ml each.
THERAPEUTIC CATEGORY:
Amino Acids and derivatives (Alimentary tract & metabolism products).
MARKETING AUTHORIZATION HOLDER:
HELP S.A. – 10, Valaoritou str., GR 144 52 Metamorphosis, Attika, Greece,
Tel.: +30.210.2815353, +30.210.2843479.
MANUFACTURED BY:
HELP S.A. – 10, Valaoritou str., GR 144 52 Metamorphosis, Attika, Greece,
Tel.: +30.210.2815353, +30.210.2843479.
WHAT YOU SHOULD KNOW ABOUT THE MEDICINE YOUR DOCTOR HAS PRESCRIBED
GENERAL INFORMATION:
L-CARNITINE is used in the treatment of primary and secondary carnitine deficiency in adults and children over 12 years of age.
Carnitine deficiency occurs when the body has a shortage either because it cannot make L-carnitine or because it cannot make enough L-carnitine.
DOSAGE AND ADMINISTRATION:
•ORAL SOLUTION: FOR ORAL ADMINISTRATION ONLY.
The Oral Solution can be drunk directly or diluted further in water or fruit juices.
Adults and children over 12 years of age:
It is advisable to monitor therapy by measuring free and acyl carnitine levels in both plasma and urine.
The management of inborn errors of metabolism:
The dosage required depends upon the specific inborn error of metabolism concerned and the severity of presentation at the time of treatment. However, the following can be considered as a general guide.
An oral dosage of up to 200mg/kg/day in divided doses (2 to 4) is recommended for chronic use in some disorders, with lower doses sufficing in other conditions. If clinical and biochemical symptoms do not improve, the dose may be increased on a short-term basis. Higher doses of up to 400mg/kg/day may be necessary in acute metabolic decompensation or the i.v. route may be required.
Haemodialysis – maintenance therapy:
If significant clinical benefit has been gained by the first course of intravenous Inestom® then maintenance therapy can be considered using 1g per day of Inestom® orally. On the day of the dialysis, oral Inestom® has to be administered at the end of the session.
MANAGEMENT OF OVERDOSAGE:
There have been no reports of toxicity from levocarnitine overdosage. Overdosage should be treated with supportive care.
What you should do in case you have omitted a dose:
If you must take the medicine continuously and have missed a dose, you should take this dose as soon as possible. If however the time for the next dose approaches, do not take the missed dose but continue your treatment normally.
EXPIRY DATE OF THIS PRODUCT:
Do not use the product after the date written on the inside and external packaging.
25°C. Protect from light.£STORAGE INSTRUCTIONS FOR THIS PRODUCT: Store at temperature
DATE OF LAST REVISION OF THIS DATA SHEET: MAY 2007.
INFORMATION ON THE RATIONAL USE OF MEDICINES
•This medicine has been prescribed by your doctor only for your specific medical problem.
•You should not give it to other individuals or use it for any other condition, without previous consultation with your doctor.
•If during your therapy there is any problem with the medicine, you should notify your doctor or pharmacist.
•If you have any questions about the information concerning the drug you are taking or if you need more information about your medical problem, do not hesitate to ask your doctor or pharmacist.
•To be effective and safe the prescribed drug should be used according to the instructions given to you.
•For your health and safety you must read carefully all the information regarding the drug you have been prescribed.
•Do not store medicines in bathroom cupboards; heat and moisture may alter them and make them hazardous to your health.
•Do not keep medicines you do not need any more, or those that have expired.
•For greater safety keep all medicines out of the reach of children.
® Ferrinemia
Iron (III)-hydroxide sucrose complex
20mg/ml Injection Solution/Concentratefor Solution for Infusion
Injection Solution/Concentrate for Solution for Infusion which will be refered to asFerrinemia throughout the rest of this document.
What Ferrinemia® contains
• The active substance is: Iron(III)-hydroxide sucrose complex
• The other ingredients are: Water for injection, Sodium Hydroxide
What Ferrinemia® looks like and contents of the pack
Ferrinemia® is a brown, non-transparent, aqueous solution.
5 ml of the solution are packed in clear/colourless or amber ampoules (type I glass).
Ferrinemia® is available in packs containing 5 ampoules.
HOW TO TAKE Ferrinemia®
Always take Ferrinemia® as your doctor has told you. If you are not sure how to take Ferrinemia®, check with your doctor or pharmacist.
Ferrinemia® should only be administered by the intravenous route by slow intravenous injection or by intravenous drip infusion which is the preferred route. The product must not be administered by intramuscular or subcutaneous injection. For intravenous infusion the 5ml ampoule (100mg iron) should be diluted in 0.9% saline. No other intravenous dilution solutions or therapeutic agents should be used.
Before receiving your first dose, you should receive a small “test dose” which may help reduce the chance of a serious reaction occurring.
The total dose of Ferrinemia® you require is given in single doses of one ampoule not more than three times per week. This may be increased to two ampoules not more than three times per week depending on the severity of your iron deficiency. Your doctor will take responsibility for calculating the appropriate dose and frequency of injections.
Children:
The use of Ferrinemia® has not been adequately studied in children and, therefore, Ferrinemia® is not recommended for use in children.
If you take more Ferrinemia® than you should
Overdosage can cause acute iron overloading which may manifest itself as haemosiderosis. Overdosage should be treated, if required, with an iron chelating agent.
If you forget to take Ferrinemia®
As this medicine is given in a hospital, it is unlikely that you will be given too little or too much, however tell your doctor if you have any concerns.
HOW TO STORE Ferrinemia®
Ferrinemia® is to be kept out of the reach and sight of children.
The product should not be used after the expiry date printed on the outer carton and label. The expiry date refers to the last day of that month.
Store in original carton. Do not store in temperatures above 25°C. The product should not be frozen. Once the ampoules have been opened they should be used immediately.
Shelf life after reconstitution
After dilution with 0.9% saline, the solution should be used immediately.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
If you have any further questions on the use of this product, ask your doctor or pharmacist
Marketing Authorisation Holder
HELP S.A., 10, Valaoritou Str., Metamorphosis 144 52, Athens, Greece
Manufacturer
HELP S.A., 10, Valaoritou Str., Metamorphosis 144 52, Athens, Greece